 Clinical Research
Clinical Research
After conducting various preclinical studies and confirming sufficient safety, the subject product is actually used in multiple clinical research facilities centered on Nagoya University based on the Clinical Research Law (enforced on April 1, 2018).
(Clinical research facility)
・ Nagoya University Hospital
・ Tohoku University Hospital
・ Osaka University Hospital
・ Jikei University School of Medicine Hospital
・ The University of Tokyo Hospital
We are currently looking for patients to participate in clinical research at these facilities.
Purpose of the clinical test
The conventional cardiac support type treatment device has two problems: 1) it is not optimized for each patient, and 2) it causes diastolic dysfunction of the right ventricle. The purpose of this study is to confirm and evaluate the safety and effectiveness of the new support net that improves on the above-mentioned problems in patients with dilated cardiomyopathy.
Specifically, the device is implanted in the heart by cardiac surgery to confirm and evaluate its safety and effectiveness. In addition to safety, we will investigate the effects of suppressing the expansion of the dilated left ventricle, improvement of cardiac function, and improvement of subjective symptoms (NYHA classification) and quality of life (QOL). The main observation period is 6 months (24 weeks) after surgery, and follow-up observation will be set up to 2 years after surgery.
Test method
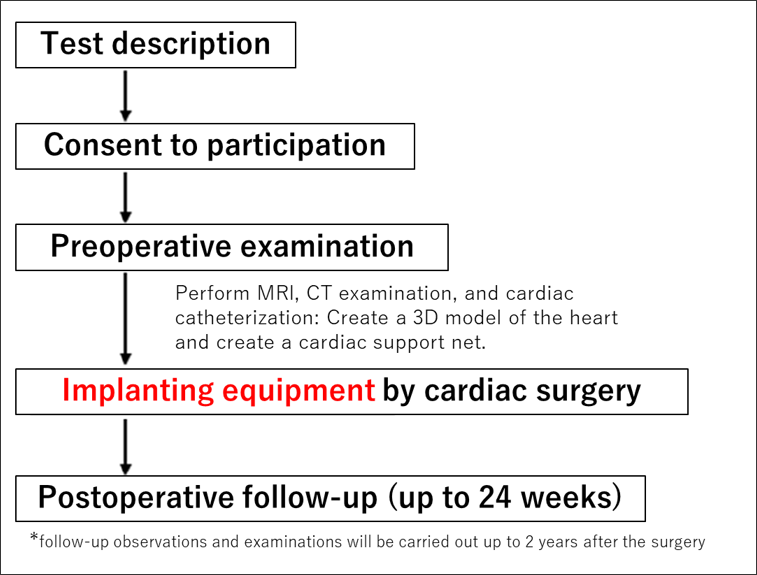
(1) Test flow
In this clinical trial, first, patients who consent to the procedure will be observed and examined to confirm their symptoms before surgery. the examinations include cardiac catheterization, MRI or contrast CT. We design the best net for you from those examined data and heart image and manufacture the test equipment as a tailor-made. After surgery, we will perform the same observations and examinations as before surgery in to confirm the safety and effectiveness of the test equipment. The evaluation and judgment will be made in the second half of the surgery (24 weeks), but follow-up observations and examinations will be carried out up to 2 years after the surgery. In addition, your survival and health condition will be investigated up to 5 years after surgery.

(2) Medical equipment (test equipment) to be used
(Problems / disadvantages of conventional similar medical devices)
1) During the surgery, the net was repeatedly cut and sewn according to the size of the patient's heart, and necessary to adjust the size. Therefore, individual differences in the net are likely to occur depending on the surgeon, and there is a risk that the pressure of the net on the heart will differ.
2) The same pressure is applied to the right and left ventricles. Therefore, applying the pressure required to prevent remodeling of the left ventricle on the surface of the left ventricle impairs the diastolic capacity of the right ventricle. It was later revealed in animal experiments and computer simulations that cardiac output was reduced.
(Outline of test equipment)
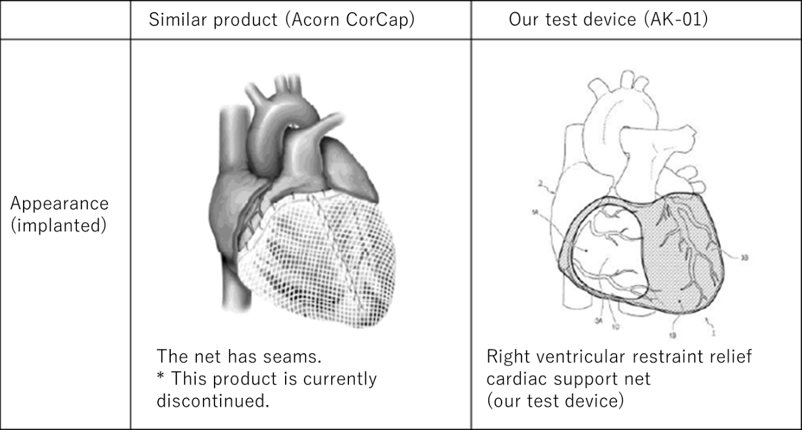
The test equipment (TaylorMade type heart shape correction net) is a mesh-like net. It is tailor-made to the patient's heart using a computer knitting machine. The polyester thread used for this net is widely used in implant medical equipment and is a biocompatible and highly safe material. Specifically, it is designed to optimize cardiac function by creating a three-dimensional cardiac shape model from a patient's cardiac MRI or CT image and combining it with cardiac catheterization data. Our test device, the cardiac support net, is significantly different from the similar medical device (CorCap) that Acorn Cardiovascular obtained in Europe in 2001 (CE-Mark) and was used in clinical practice. The differences are as follows. Our products are designed and manufactured by the tailor-made method, which eliminates the need for intraoperative adjustment, and the open structure of the right ventricle prevents deterioration of the diastolic function of the right ventricle. The structure is such that sufficient pressure is applied to the left ventricle. Therefore, by implanting this net on the heart, it can be expected to have the effect of suppressing the progression of cardiac enlargement (cardiac remodeling) in the left ventricle, which is the main cause of worsening heart failure, and improving cardiac function.
The figure below shows a similar medical device (Acorn CorCap) and our test device implanted on the heart.
(3) Surgical operation for test equipment
A cardiac surgeon with solid experience will surgically implant the test equipment.
In surgery, the skin of the chest is first incised under general anesthesia, the sternum is incised vertically, the pericardium that surrounds the heart is incised, and then a test device (heart support net) is implanted on the surface of the heart. The upper end of the heart (base of the heart) and the upper end of the net are sutured with a thread so that the perforated part of the right ventricle does not drift. Implantation of a test equipment takes about 30 minutes. After installation, echocardiography and biventricular pressure measurements are performed to evaluate the effectiveness of the cardiac support net. As a result, if it is determined that cardiac output has decreased, it will be changed to a larger support net. Evaluate the function of the heart, and if there is no problem, partially close the pericardium, re-suture the sternum, close the wound, and finish the operation. Except for the implantation of a heart net, the procedure is the same as for regular heart surgery. No artificial heart-lung machine is used in this surgery. The epicardium and test equipment usually adhere and integrate within or about 4 weeks, so it will be difficult to remove after that. Therefore, once the test equipment is implanted, it will not be removed in principle. It will continue to function to prevent progressive cardiac enlargement during the patient's lifetime. However, if infection occurs during the hospitalization period after surgery and it is determined that the test equipment needs to be removed, the test equipment may be removed. Infectious mediastinitis and folliculitis usually develop in or about 2 weeks after surgery. At this stage, it is considered relatively easy to remove the test equipment. The risk of developing infection varies depending on the preoperative condition and hospital, but such risk is considered very low since the operation time of this test device is relatively short.
(4) Postoperative course
After surgery, patients generally move to the intensive care unit (heart center) as they awaken from anesthesia and regain consciousness. Family members can meet with patients at the Heart Center (cardiovascular intensive care unit) within 1 to 2 hours after surgery. The patient's body is connected to a catheter and tracheal tube for artificial respiration to ensure safety and to effectively recover from surgery. While in the Heart Center, a biometric monitor monitors the patient's heart rate, blood pressure, and body temperature. Particular attention is paid to the exacerbation of heart failure and the development of new arrhythmias. If a fatal arrhythmia occurs, antiarrhythmic drug treatment is performed, and the use of an implantable defibrillator should be considered. Generally, the length of stay at Heart Center is 1-2 days, and the length of hospital stay is expected to be around 14 days, it depends on patient’s condition. During hospitalization, some discomfort may occur in or on the incision area of surgery. For patients who feel strong pain, we will prescribe drugs to relieve the pain.
(5) Problems considered in the remote period
Implanting a net can/may make difficult for future coronary artery bypass grafting. This test device is indicated for patients with idiopathic dilated cardiomyopathy and excludes patients who have undergone coronary artery bypass grafting in the past or who are scheduled for coronary artery bypass grafting. Therefore, it is unlikely that coronary artery bypass grafting will be required after implantation of this test device. In the case of the conventional product (Acorn CorCap) clinical trial, there was a case reported about heart transplantation after implantation, but the adhesion between the pericardium and CorCap was highly fixed to peel off, and leaving CorCap on the pericardial side, and then proceeded the heart transplantation. Therefore, the amount of bleeding may increase during a heart transplant. There is also a report that an implantable ventricular assist device was attached, and in this case, it was reported that CorCap strengthened the heart surface and made it easier to fix the devascularization of the ventricular assist device. Therefore, we believe that there is little impact on the risk of installation of ventricular assist device.
Clinical trial schedule
The clinical trial will be conducted according to the "Main test schedule" below.
After consenting to participate in the clinical trial, we will perform a preliminary examination and diagnostic imaging to confirm if the patient can participate in the clinical trial. If similar tests and diagnostic imaging have been performed before consent, the data may be used for confirmation purpose. Since surgical operation is required to implant the test equipment, hospitalization is essential for the treatment. The postoperative visit schedule is 4 weeks, 12 weeks, 24 weeks, 1 year, and 2 years after the operation. In addition, we will check patient’s survival and health condition up to 5 years after surgery. Even if to stop participating in the test, the patient will still be required to undergo an examination to confirm the health and safety.

The tests performed are to confirm the safety and effectiveness of the test equipment. There is no special test for this clinical trial. The examinations are just the commonly performed. However, the number of tests will be slightly higher than in normal practice, such as the number of cardiac catheterization tests and simultaneous myocardial biopsy (which is optional depending on your condition). In addition to those listed here, additional tests may be performed if a doctor deems it necessary due to patient’s conditions.
The examinations and purposes are as follows.
1) Inspection for safety
- ・ Vital signs: Measures body temperature, blood pressure, and heart rate.
- ・ 12-leads electrocardiography: Records the heart rhythm.
- ・ Holter ECG: Records a 24-hour heart rhythm to monitor arrhythmias.
- ・ Blood test: Evaluates anemia, inflammatory reaction, renal function and liver dysfunction.
- * General blood test: WBC, white blood cell fraction (neutrophils, lymphocytes, monocytes, eosinophils, basophils), RBC, HB, HT, PLT
- * Blood biochemical tests: CK, CK-MB, CRE, AST, ALT, γ-GTP, T-Bil, ALP, LDH, BUN, TP, Alb, UA, TG, T-Cho, HDL-C, LDL- C, GLU, Na, K, Cl, CRP
- * Blood coagulation/fibrinolysis system test: PT, APTT, FBG (conducted only before surgery) * BNP
- ・ Imaging diagnosis (MRI or CT, chest X-ray, echocardiography, cardiac RI examination, cardiac catheterization)
- * The safety and effectiveness of the test equipment AK-01 will be confirmed by diagnostic imaging tests.
2) Examination for judgment of surgical indication and confirmation of effectiveness
- ・ NYHA classification and INTERMACS Profile survey: Confirm improvement of subjective symptoms.
- ・ Chest X-ray examination: Calculates cardiothoracic ratio and confirms prevention of cardiac remodeling.
- ・ Respiratory stability test: A test is performed at the same time as the Holter ECG test to check the respiratory condition.
- ・ Echocardiography (transthoracic wall): Calculates left ventricular ejection fraction (LVEF) and confirms improvement in cardiac function.
- ・ Transesophageal echocardiography: Used intraoperatively to evaluate cardiac function before and after cardiac net implantation.
- ・ BNP: A blood test confirms improvement in the severity of heart failure.
- ・ 6-minute walking test: The maximum walking distance in 6 minutes is measured to confirm the improvement of athletic/physical ability.
- ・ Cardiopulmonary exercise load test: Confirm the improvement of athletic ability by biking.
- ・ QOL evaluation: Fill in the results on the Minnesota Questionnaire and EQ-5D Questionnaire to confirm improvement in quality of life.
- ・ Cardiac catheterization: Measures the pressure and cardiac output of the right and left ventricles to evaluate cardiac function.
- ・ Myocardial biopsy: A small piece of heart muscle is scraped off with special forceps and observed under a microscope to confirm the diagnosis and condition of dilated cardiomyopathy. It will be an optional inspection.
- ・ Coronary angiography: Checks preoperatively for stenosis in the coronary arteries that feed the heart.
- ・ Heart RI test: A small amount of radioactive isotope (isotope) is injected into a blood vessel to evaluate cardiac function, the state of the sympathetic nerve that controls the heart, and cardiomyocyte damage.
Drugs / treatments restricted during the study
Testing equipment does not replace medication, diet, exercise, etc. The purpose is to prevent the worsening of heart failure and improve cardiac function by treatment using test equipment. As was done before participating in the study, it is necessary to continue the management of heart failure such as drug treatment, diet and exercise therapy.
<Prohibited therapy in combination>
It is prohibited to perform other heart surgery during the surgery for the test equipment.
<Concomitantly prohibited drugs>
No concomitantly prohibited drugs have been set. Patient can continue to take the cardiac treatment drug that you have been taking before the test. Depending on the postoperative symptoms, the cardiac treatment drug may be reduced or changed at the discretion of the doctor in charge.
© iCorNet Laboratory All rights reserved.




