 Participation in Clinical Research
Participation in Clinical Research
Implementation period and number of participants
This clinical study will be conducted from September 2019 to December 2021 (follow-up period of 2er4ree years after surgery), and a total of 3 patients will participate in all facilities.
Participants in clinical research
Participants in this clinical study are those who meet the following conditions:
- ・ 20 years old or older and 75 years old or younger who have been diagnosed with dilated cardiomyopathy
- ・ Patients with continuous heart failure symptoms despite being treated with drugs for heart failure ・NYHA classification is III or IV and Level 4 to 7 of INTERMACS Profile
- ・ Patients with cardiac enlargement with a left ventricular end-diastolic diameter of 60 mm (30 mm / m2) or more by echocardiography
- ・Decreased left ventricular ejection fraction and cardiac function on echocardiography
- ・ Patients with RVEDVi ≤ 170 mL / ㎡ by MRI or contrast-enhanced CT examination (in the case of right ventricular restraint reduction type)
In addition, you may not be able to participate in this clinical study if any of the following applies.
- ・ Patients with excessive cardiac enlargement with a terminal diameter of left ventricular dilatation exceeding 85 mm
- ・ Patients with a left ventricular ejection fraction of less than 10% and severely impaired left ventricular function
- ・ Patients who have undergone cardiac surgery in the past (excluding implantable cardioverter-defibrillators and pacemaker implantable surgery)
- ・ Patients planning other cardiac surgery
- ・ Patients with a history of coronary artery bypass graft surgery (CABG) or who are scheduled to undergo CABG
- ・ Patients who have undergone percutaneous coronary intervention (PCI) or trans-myocardial laser revascularization within 3 months before surgery or candidates for it
- ・ Patients undergoing intra-aortic balloon pumping
- ・ Patients who have a history of heart transplantation or wish to have a heart transplant
- ・ Patients with NYHA classification IV who are wearing an auxiliary heart device or who are scheduled to undergo surgery on the auxiliary heart device.
- ・ Patients who develop acute myocardial infarction, unstable angina, or cerebrovascular accident within 3 months before surgery
- ・ Patients who have undergone biventricular pacing (including implantable cardioverter-defibrillator and pacemaker implant surgery) within 3 months before surgery, or who plan to perform it in the future
- ・ Patients with hypertrophic cardiomyopathy
- ・ Patients with active infections
- ・ Patients with severe liver dysfunction, renal failure, or lung function problems
- ・ Persons with widespread peripheral vascular disease
- ・ Patients with central nervous system diseases (including cerebral infarction and cerebral hemorrhage) with poor prognosis
- ・ Patients who are refused blood transfusion
- ・ Patients with malignant diseases with poor prognosis such as cancer
- ・ Those who having severe dementia and drug addiction People with severe allergies
- ・ Those who are pregnant, breastfeeding, or may become pregnant
- ・ Those who are participating in other clinical trials (including clinical research)
There are several other criteria for participating in clinical research. In addition, even after consenting to participate in a clinical study, you may not be able to participate depending on the results of prior tests to see if the criteria are met.
Expected effects and disadvantages
(1) Expected major effects
- ・Major effects expected according to animal tests
In a large animal model of chronic heart failure (dogs, pigs), when compared with the control group (group without net) and the normal net group (group with net without an opening in the right ventricle), the test equipment (cardiac support net with an opening on the right ventricle) indicated that the contractility of the right and left ventricles was significantly improved. In cardiac simulations in a chronic heart failure model using a supercomputer, our test equipment (a heart support net with a perforated right ventricle) increases cardiac output and reduces energy consumption of the left ventricular myocardium and found to be effective and improve the energy efficiency of the left ventricle. In the state of heart failure, the orientation of myocardial fibers is disturbed and the contraction efficiency is reduced, but simulations have shown that the disorder is improved by implanting test equipment. These results indicate that this test device can be expected to improve cardiac function immediately after implantation.
- ・Major effects expected from similar medical devices
According to the results of overseas clinical trials of similar medical devices devised overseas, these devices reduce the size and capacity of the left ventricle, improve the ejection fraction of the left ventricle, and change the heart to a more elliptical shape. It is also expected to reduce the needs for major heart surgery required for the progression of heart failure, and improve the patient's quality of life (QOL).
The following is a summary of the results of overseas clinical trials using similar medical devices.
* Reference: "Clinical Evaluation of the CorCap Cardiac Support Device in Patients With Dilated Cardiomyopathy" 2007, The Society of Thoracic Surgeons
: "Clinical evaluation of CorCap cardiac assist device for patients with dilated cardiomyopathy" 2007 American Thoracic Surgery Society (Elsevier)
A total of 300 patients participated. There were 148 patients treated with similar medical devices (test device group) and 152 patients not treated with similar medical devices (control group). Some of these patients have undergone mitral valve repair and replacement.
The table below summarizes the results of post-treatment changes in the NYHA classification that determine the severity of heart failure and the percentage of patients who have not undergone major cardiac surgery after treatment. Patients treated with similar medical devices (test device group) had a higher improvement rate of NYHA classification at 45%, and those who had not undergone major cardiac surgery had a higher rate of 89%, and the effect was high.
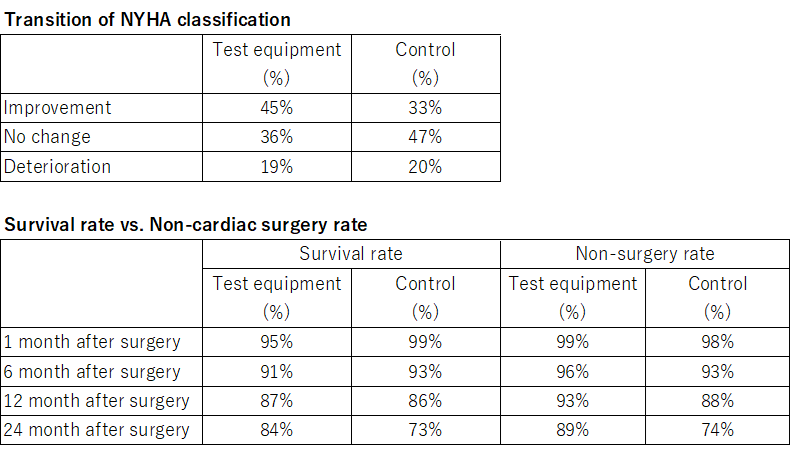
(2) Expected major disadvantages
- ・Expected malfunction of test equipment
After the implantation surgery, the net of the test equipment may have deteriorated and the net may have torn, but in the 100 million endurance test (equivalent to the heart rate for 3 years), no deterioration or tear of the net was observed. In fact, within a few months after surgery, the net is expected to adhere to your heart and stabilize its strength.
- ・Expected complications
Complications include perioperative (immediately after implantation) infections (wound infections, pneumonia, catheter infections, etc.), bleeding, worsening heart failure, and effects on coronary arteries (blood vessels around the heart). In the distant period (sometime after implantation), constrictive pericarditis may occur.
Wound infection: An infection that occurs at the surgical site. It can happen at a certain frequency. In general, it has been reported that surgery to transplant artificial objects increases the likelihood of infection. The cardiovascular surgery department of the research facility has taken various preventive measures against wound infections and is doing its best to prevent wound infections.
Effects on coronary arteries: The test device (cardiac support net) works by compressing the surface of the heart. Therefore, there is a concern that the coronary arteries may be compressed and the blood flow in the coronary arteries may be reduced. In this regard, animal studies have been conducted to confirm that implantation of test equipment does not affect the blood flow reserve or morphology of the coronary arteries.
Constrictive pericarditis: Since a test device (foreign body) is attached around the heart, there is a concern that constrictive pericarditis may occur in the remote period, where the surface tissue of the heart becomes fibrotic or calcified. However, it has been reported that there was no occurrence of constrictive pericarditis in a 5-year clinical trial of Acorn CorCap, a similar medical device of this study device. In addition, in the long-term implantation test in animals with our test equipment, the tissue reaction and adhesion around the heart were slight.
Other common adverse events that may occur in connection with heart surgery include: Allergic reaction, arrhythmia, cardiac tamponade, chronic pain, death, cardiogenic shock, deterioration of hemodynamics that may lead to neuropathy, infection / sepsis, local skin reaction, myocardial infarction, surgical disorder, epicardial fluid , Pericarditis, pneumothorax, disorders leading to lung / kidney / liver failure, reoperation, thromboembolism
The table below shows the results in the same literature as in "Overseas clinical trials with similar medical devices" in "(1) Expected major effects". It shows the number of serious adverse events among the adverse events (all unfavorable events that occurred during clinical trial participation) during the entire observation period. Among them, there was no event (side effects) suspected to be caused by similar medical devices.
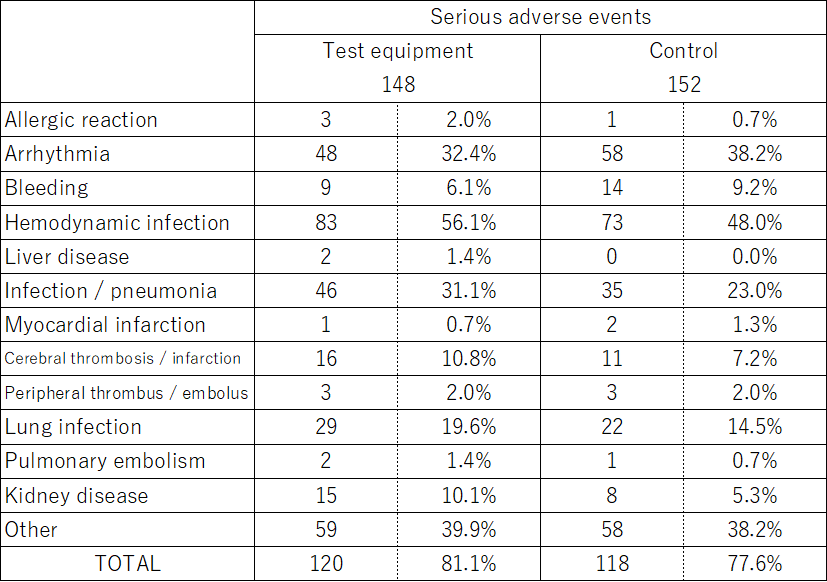
Furthermore, as of December 2004, 174 patients in five European countries (France, Germany, the Netherlands, Sweden, and Italy) have a track record of using similar medical devices. Even in such patients, no adverse events suspected to be caused by similar medical devices have been reported. These adverse events have been reported so far, and not all adverse events occur in all patients. The doctor in charge will closely observe the expected adverse events and symptoms as described above, and provide appropriate treatment each time.
Cases where participation in the exam is canceled
After participating in this clinical trial, the patient may be discontinued from participating in the trial if any of the following occurs. Even in that case, please be assured that we will provide appropriate treatment for you.
- 1. When it is judged that the test equipment cannot be used at the time of surgery and it is not implanted
- 2. When you or your family offer to cancel your participation
- 3. If you cannot come to the hospital
- 4. If the patient fails to follow the rules
- 5. If your doctor determines that you have an unpleasant adverse event and it is difficult to continue the study.
- 6. If your doctor determines that you need to discontinue this study for any other reason
Even if participation in the test is canceled, we will conduct medical examinations and tests as much as possible to confirm the safety at the time of cancellation. Please note that once the test equipment is implanted, it will not be removed in principle.
Correspondence after the test
If the adverse events that occurred during the study period did not recover and continue, in principle, our follow-up will be conducted until the symptoms recover or stabilize. If any adverse events that may be related to the test equipment are found after the test, follow-up will be conducted.
In case of test-related health hazards
This clinical trial is scientifically planned based on the results of previous studies and trials, and patient safety is our top priority.
The treatment performed this time is not the standard treatment for dilated cardiomyopathy. However, we believe that this clinical trial can be expected to have effects such as suppressing the expansion of the dilated left ventricle and improving cardiac function. If this test causes any health hazard, we will provide the best treatment within the scope of normal medical care. In addition, we take out compensation insurance to provide appropriate compensation for health hazards. In addition, we take out clinical research insurance in case of unexpected serious adverse events that are suspected to be caused by the test equipment and leave aftereffects.
Cost burden in clinical trials
Each facility will bear the cost of the surgery to attach the cardiac support net. All planned in-hospital examinations, cardiac support net installation surgery costs and hospitalization costs will be covered by research expenses. Medical insurance will cover the cost of medical treatment after discharge.
Providing inspection data to the outside
- 1. The test equipment is tailor-made and designed and manufactured. Therefore, your preoperative examination data (12-lead electrocardiography, chest X-ray, echocardiography, MRI / contrast CT, cardiac catheterization) will be provided to Nagoya University.
- 2. Your preoperative, intraoperative, and 24-week postoperative examination data (12-lead electrocardiography, echocardiography, MRI / contrast CT, cardiac catheterization) will be provided to Nagoya University to simulate cardiac function. The inspection data will be sent to UT-Heart Laboratory Co., Ltd. A cardiac function simulation is performed there. The effect of the cardiac support net is estimated by analyzing the degree of improvement in cardiac function when the cardiac support net is implanted with a supercomputer. It will be different from the results compiled in this trial, but will be conducted as ancillary research to help future device design.
- 3. When the test data is provided to Nagoya University, personally identifiable information such as your name will not be included.
Privacy protection
- 1. The confidentiality of the participants will be kept and no name or personally identifiable information will be disclosed.
- 2. The information obtained in this test may be provided to joint research institutes or published in related academic societies and medical journals. However, all information will be reported using the code number, so no information that identifies your name or individual will be disclosed.
- 3. The test equipment provider and related persons involved in this test, the person in charge of cardiac function simulation, the person in charge of the committee of this hospital and the person in charge of the government (Ministry of Health, Labor and Welfare) who evaluate this test may view your medical record. There is. However, privacy is still protected.
- 4. By agreeing to participate in this clinical trial and signing the consent document, it is considered that the above persons are allowed to view materials such as medical records.
- 5. Please note that if the clinical trial is discontinued in the middle of the process, we will also report the results of the clinical trial conducted before the discontinuation and your situation after the discontinuation. Please be assured that your privacy will be protected even in such case.
What we want patients to comply
- 1. If you have any other medications you are currently using or have other treatments, be sure to tell your doctor about the treatment and consult with your doctor to see if you can continue those treatments.
- 2. If you visit other medical institution, add new medicine, or change it, be sure to inform your doctor about the type and amount of medicine and the period of use.
- 3. If you want to use other medicines (including over-the-counter medicines such as Chinese herbs and supplements), please consult your doctor in advance.
- 4. Please follow your doctor's instructions until the end or discontinuation of the clinical trial.
- 5. If for some reason you want to stop or suspend your visit, please inform your doctor. If you cancel without permission, your doctor may contact you.
- 6. If you feel any abnormality during the clinical trial period, please contact your doctor or the responsible doctor immediately.
© iCorNet Laboratory All rights reserved.




