MORE OPTIONS
FOR CARDIOMYOPATHY
拡張型心筋症の治療に
投薬と移植以外の選択肢を。
心臓形状矯正ネットの研究・開発を行う
iCorNet研究所
NEWS
お知らせ
-
「致死性不整脈に対する電導性繊維電極付きネットによる意... 続きを見る
2023.04.19
おしらせ -
秋田先生が答えるQ&Aが追加されました
2023.04.11
おしらせ -
弊社のインタビュー記事がJETROのホームページに掲載... 続きを見る
2023.03.31
おしらせ -
弊社の『特発性拡張型心筋症に対する新たな治療法(心臓形... 続きを見る
2023.03.28
おしらせ -
秋田先生が答えるQ&Aのページを開設しました
2022.10.19
おしらせ
for PATIENTS
患者さん・一般の方へ
いつも通りを1日でも多く。
進行を遅らせる形状矯正ネット
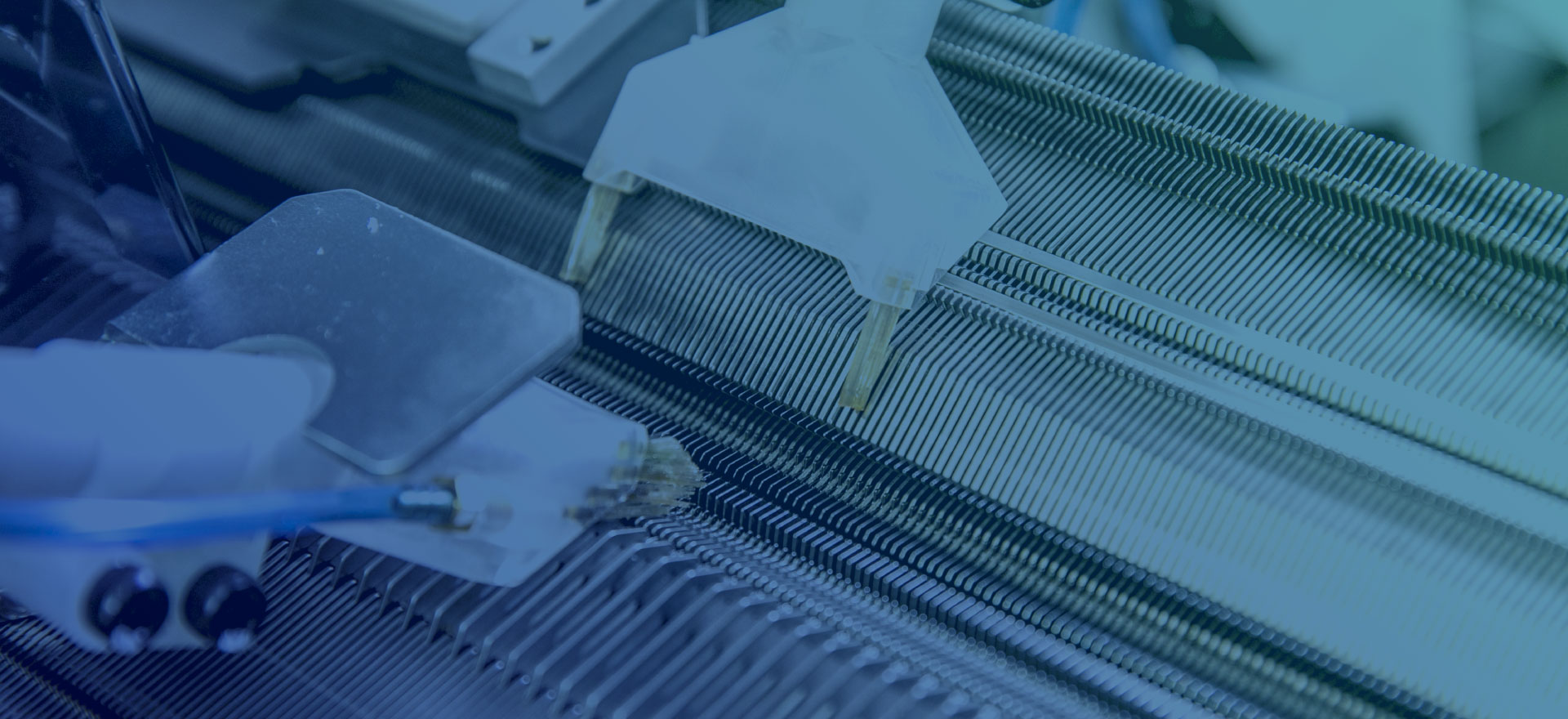
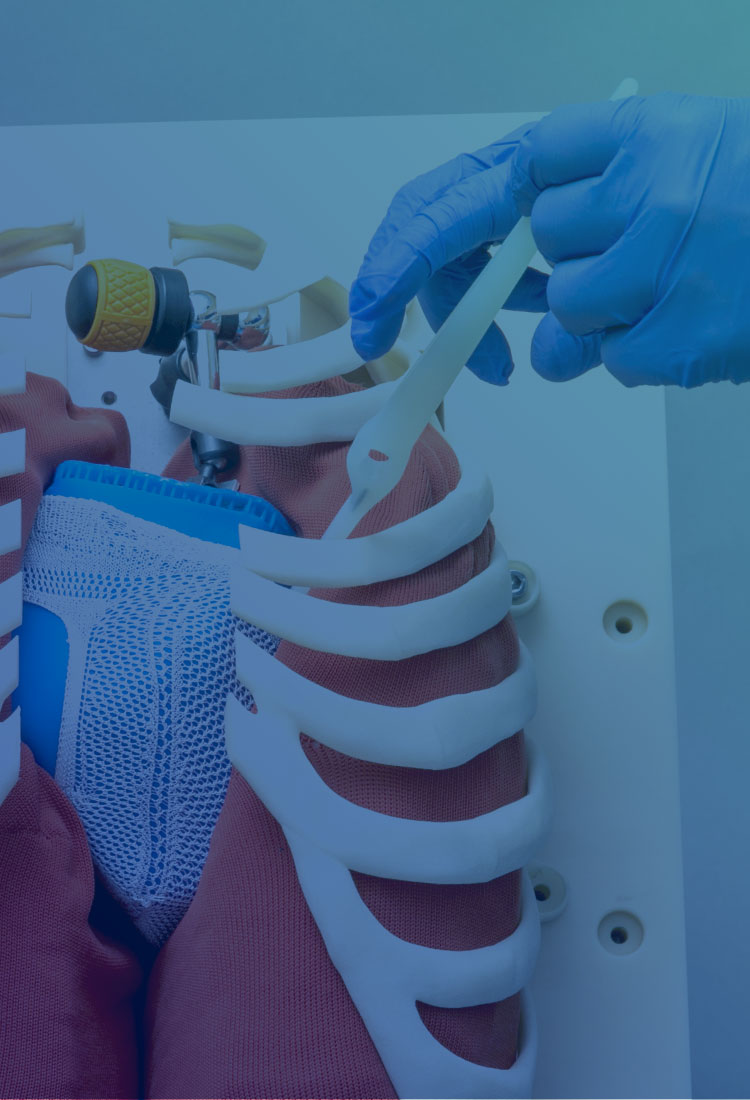
iCorNet研究所では、特発性拡張型心筋症(拡張型心筋症)の進行を遅らせる心臓形状矯正ネットを研究・開発しています。
診断を受けたご本人様やご家族のみなさまへ向けて、拡張型心筋症という病気について、私たちが研究・開発している心臓形状矯正ネットがどのようなものなのかをお伝えします。
for MEDICAL
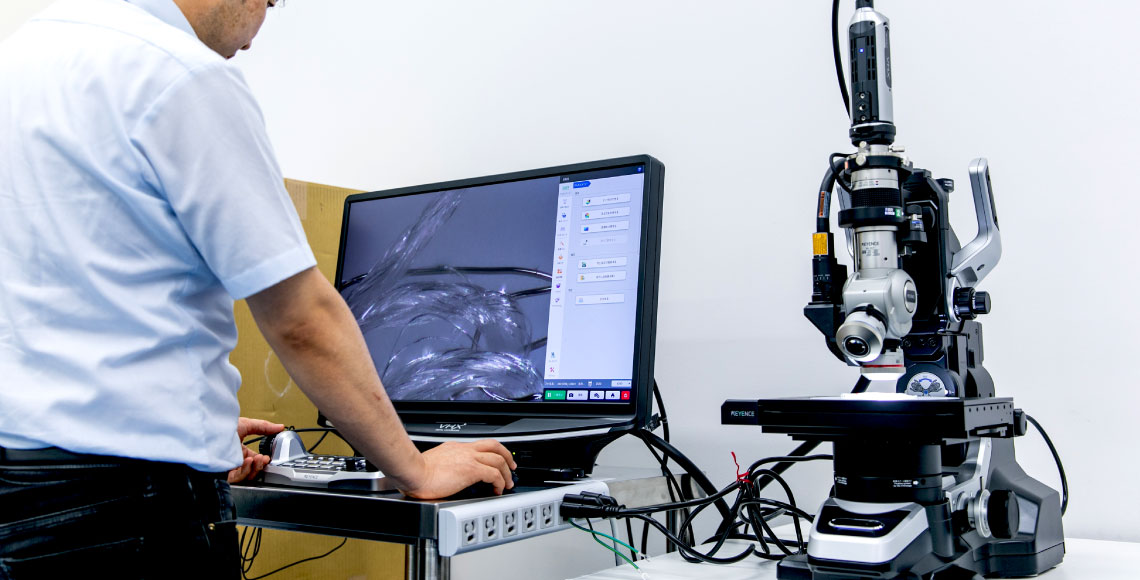
医療従事者の皆様へ
心不全治療の現場に
世界最高レベルの技術で応える
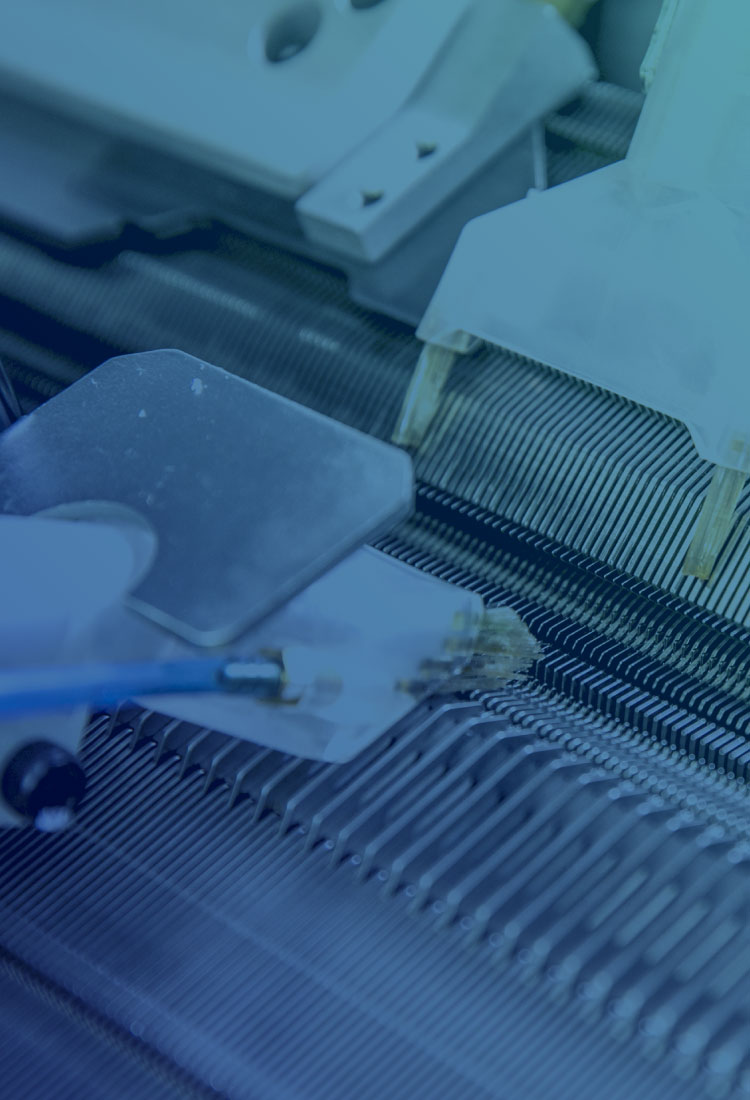
心臓形状矯正ネットは、特発性拡張型心筋症(拡張型心筋症)の治療において、投薬・補助人工心臓・心臓移植手術以外の有効な手段として期待できる製品です。
世界最高レベルの技術力を誇るUT-Heart研究所との協力のもと、患者に合わせたテーラーメイド方式や、経年劣化によるほつれのリスクがない完全無縫製の適応により、長期の耐久性を実現しました。

About us
iCorNet(アイコアネット)
について
名古屋大学発ベンチャー(038号)
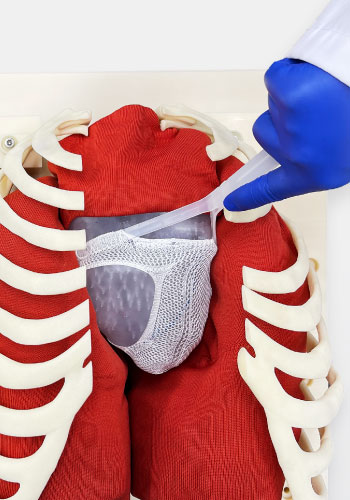

拡張型心筋症の
新しい解決策を開拓する
拡張型心筋症は完治が難しい病気です。現在、投薬による苦痛の軽減か、心臓移植しか有効な手段がないと言われています。そんな中、「患者さんが元気に過ごせる時間を少しでも長くしたい」という思いから、新しい対処法の選択肢として“心臓形状矯正ネット”の研究を始めました。

CONTACT
お問い合わせ
心臓形状矯正ネット治療についてや、
拡張型心筋症のことなど
なんでもお気軽にお問い合わせください。