OUTLINE
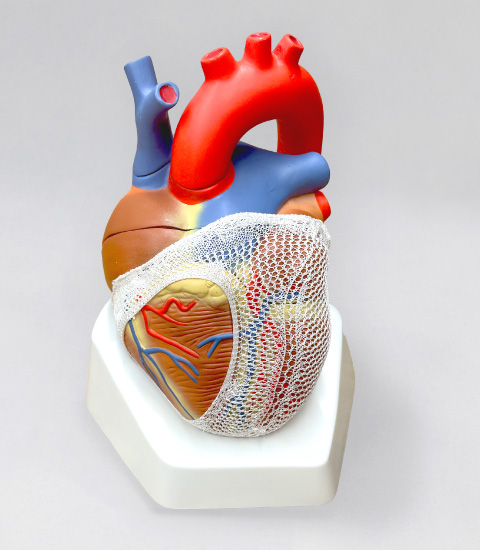
心臓形状矯正ネットFAB
Background
Research Background
In the treatment of heart failure
The Way to Solve the Biggest Challengestowards (a destination)
Despite treatment with a variety of therapies, the number of patients with severe heart failure is rapidly increasing. The cost of medical care has become a major burden on the healthcare economy, and there is an urgent need to develop new, inexpensive, and widely applicable treatment methods.
In the past, Acorn CorCap, a cardiac support therapy that covers the heart with a mesh-like bag to prevent cardiac dilation (cardiac remodeling phenomenon), obtained CE Mark and was actually used for treatment in Europe. In patients with severe heart failure, progressive cardiac dilation worsens heart failure, so it was hoped that this treatment would work to reduce the dilation (cardiac remodeling).
However, the size of the net had to be adjusted intraoperatively by the surgeon to match the six standard sizes to the size of the individual patient's heart. In addition, since both ventricles were compressed with equal force, if enough pressure was applied to the left ventricle, the right ventricle would be overcompressed, resulting in right ventricular diastolic dysfunction and reduced cardiac output. The product was not approved in the U.S. due to a lack of evidence of effectiveness in terms of life expectancy.

Improved on past shortcomings.
新しい心臓形状矯正ネットDevelopment of
本研究代表者らは2027年からの3年間テルモ生命科学芸術財団臨床研究助成を受け特定臨床研究、2022年からの3年間 AMED橋渡し研究加速ネットワークの補助を受け医師主導型探索的治験を実施した。
また2023年からの3年間は東京都先端医療機器アクセラレーションプロジェクト(AMDAP)の補助を受け、GLP基準に沿った非臨床試験を実施した。
これらのプロセスを経て「術中の調整が不要」「右室拡張の障害にならない」といった過去の心臓サポート型治療の問題点を解決する「心臓形状矯正ネット(テーラーメイド方式心臓形状矯正ネット)」による治療の開発を行ってきた。
As a result, the following results have been achieved
(1) Establishment of a method for creating 3D heart models from cardiac images of patients with heart failure.
②島精機製コンピュータ編み機用心臓形状矯正ネット型紙作成プログラムの開発
③心臓形状矯正ネットの試作と慢性心不全大型動物モデルでの心臓形状矯正ネットの安全性・有効性の検証
(iv) In 2019-2021, three multicenter clinical studies (First in Human) were conducted in accordance with the Clinical Research Act, which showed significant improvement in motor performance.
➄臨床研究の結果を基に2022-2025年名古屋大学を拠点とした医師主導型探索的治験を実施した。
⑥2023-2025年名古屋大学を拠点とした医師主導型探索的治験を実施し、心臓形状矯正ネットの安全性、有効性の評価を行い、好結果を得た。
⑦AMDAPの補助を受けGLP基準での非臨床試験を実施した

Overview
Research Overview
in patients with dilated cardiomyopathy.
心臓形状矯正ネットの臨床POC
jRCTs042180025:拡張型心筋症に対するテーラーメイド方式心臓形状矯正ネットの臨床試験
The primary endpoint is to evaluate the safety of the study device, but efficacy in terms of improvement of cardiac function, prevention of left ventricular remodeling, and improvement of quality of life will also be evaluated in an exploratory manner as secondary endpoints.
01Main selection criteria
| Target Patients (Target Diseases) | Patients with dilated cardiomyopathy presenting with heart failure and NYHA classification III or IV whose symptoms do not improve with current treatment (excluding patients who are planned for concomitant surgery such as mitral valve replacement, mitral valve plication, or tricuspid valve plication) |
|---|---|
| selection criteria |
Patients who meet all of the following criteria will be selected as subjects Patients who have given written consent to participate in the study of their own free will Patients who are at least 20 years old and less than 75 years old at the time of consent Patients with persistent heart failure symptoms despite at least 3 months of optimal medical therapy for heart failure (ACE inhibitors, ARBs, diuretics, beta-blockers, oral inotropic agents, etc.) and no improvement with current therapy. Patients with NYHA Classification III or IV and Level 4 to 7 of INTERMACS Profile Patients with LVEDD≥60mm or LVEDDi≥30mm/m2 on echocardiography Patients with LVEF ≤ 351 TP3T on echocardiography or RI Patients with RVEDVi ≤ 170 mL/m2 on MRI or contrast CT scan Patients who are willing to undergo follow-up examinations and observations and who are able to visit the implementing institution. |
| exclusion criteria |
Patients who meet any of the following criteria should be excluded Patients with excessive cardiac enlargement (LVEDD > 85 mm) Patients with severely depressed left ventricular function (LVEF <10%) Patients with a history of previous cardiac surgery (except for patients with pericardial effusion and minimal pericardial adhesion, and excluding ICD and pacemaker implantation surgery) Patients scheduled for other cardiac surgery Patients with a history of CABG or scheduled for CABG Patients or candidates for PCI or transmyocardial laser revascularization within 3 months prior to surgery Patients undergoing IABP Patients with NYHA Classification IV who are candidates for assisted cardiac device and heart transplantation. Patients who have undergone biventricular paging (CRT (including ICD and pacemaker implantation surgery) or are scheduled to undergo CRT within 3 months prior to surgery. Patients expected to live less than 1 year ・Last stage heart failure patients with increased surgical risk |
02research methods
- 各共同研究施設(5大学)において同意の得られた対象患者に対して、心臓画像およびカテーテル検査より設計されたテーラーメイド方式心臓形状矯正ネットを作成し、全身麻酔下に装着する。
- Cardiac function, cardiac remodeling effects, quality of life, and exercise tolerance will be evaluated at 6 months to assess adverse events and efficacy.
- Nagoya University will be in charge of the Clinical Trials Secretariat.

CONTACT
Contact Us
心臓形状矯正ネット治療についてや、
拡張型心筋症のことなど
Please feel free to contact us for any inquiries.
